ANSI AAMI ISO 11135-2014 pdf download ANSI AAMI ISO 11135-2014 pdf download.Sterilization of health care products — Ethylene oxide — Requirements for development, validation and routine control of a sterilization […]
Read more
ANSI standards list

ANSI AAMI ISO 11135-2014 pdf download ANSI AAMI ISO 11135-2014 pdf download.Sterilization of health care products — Ethylene oxide — Requirements for development, validation and routine control of a sterilization […]
Read more
ANSI AAMI ISO 11134-1993 pdf download ANSI AAMI ISO 11134-1993 pdf download.Sterilization of health care products – Requirements for validation and routine control – Industrial moist heat sterilization 1 Scope […]
Read more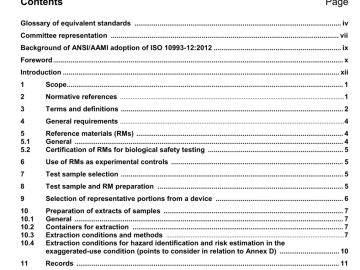
ANSI AAMI ISO 10993-12-2012 pdf download ANSI AAMI ISO 10993-12-2012 pdf download.Biological evaluation of medical devices 5.1 General Reference materials (RMs) are established by individual laboratories. The extent of chemical, […]
Read more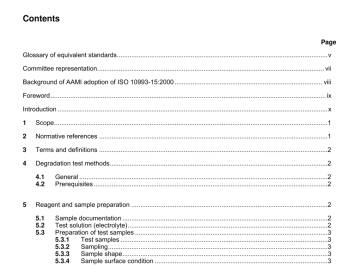
ANSI AAMI ISO 10993-15-2000 pdf download ANSI AAMI ISO 10993-15-2000 pdf download.Biological evaluation of medical devices 1 Scope This part of ISO 10993 provides guidance on general requirements for the […]
Read more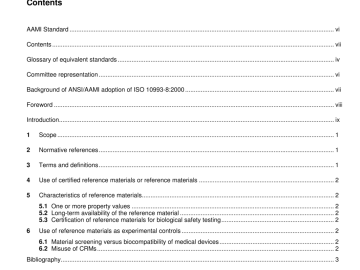
ANSI AAMI ISO 10993-8-2000 pdf download ANSI AAMI ISO 10993-8-2000 pdf download.Biological evaluation of medical devices 1 Scope This part of ISO 10993 specifies requirements on the use of reference […]
Read more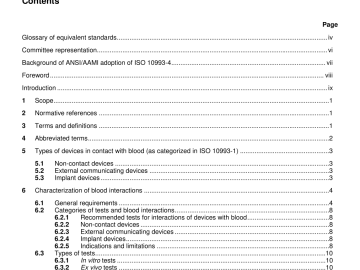
ANSI AAMI ISO 10993-4-2002 pdf download ANSI AAMI ISO 10993-4-2002 pdf download.Biological evaluation of medical devices 6.1.1 Figure 1 illustrates a decision tree that can be used to determine whether […]
Read more
ANSI AAMI ISO 8836-2015 pdf download ANSI AAMI ISO 8836-2015 pdf download.Suction catheters for use in the respiratory tract This annex provides a concise rationale for the important requirements of […]
Read more
ANSI AAMI ISO 7199-2016 pdf download ANSI AAMI ISO 7199-2016 pdf download.Cardiovascular implants and artificial organs — Blood-gas exchangers (oxygenators) 1 Scope This document specifies requirements for sterile, single-use, extracorporeal […]
Read more
ANSI AAMI ISO 7198-2016 pdf download ANSI AAMI ISO 7198-2016 pdf download.Cardiovascular implants and extracorporeal systems—Vascular prostheses—Tubular vascular grafts and vascular patches 1.1 This International Standard specifies requirements for the […]
Read more
ANSI AAMI ISO 5841-2-2014 pdf download ANSI AAMI ISO 5841-2-2014 pdf download.Implants for surgery — Cardiac pacemakers 1 Scope This part of ISO 5841 specifies requirements for reports on the […]
Read more